Off-Brand Ozempic: FDA Enforcement And Potential Drug Shortages
Table of Contents
The Rise of Counterfeit and Off-Brand Semaglutide
The increased demand for semaglutide stems from its effectiveness in both weight loss and diabetes treatment. Ozempic, a brand-name semaglutide injection, has gained significant media attention for its weight-loss benefits, leading to a surge in demand that far outstrips supply. This high demand creates a lucrative black market for counterfeit medications, with unscrupulous manufacturers and distributors capitalizing on the desire for quick weight loss and improved glycemic control.
The dangers of using unapproved semaglutide products are substantial:
- Unknown purity and dosage: Counterfeit medications often contain inaccurate dosages of the active ingredient or may contain none at all. This means patients may not receive the expected therapeutic benefits or may be exposed to unexpectedly high doses, leading to adverse effects.
- Potential for harmful contaminants: Off-brand Ozempic produced in unregulated facilities may be contaminated with dangerous substances, including heavy metals or bacterial toxins, posing severe risks to health.
- Lack of efficacy guarantees: Without the rigorous testing and quality control of FDA-approved medications, there's no guarantee that off-brand semaglutide will work as intended. Patients may waste time and money on ineffective products.
- Risk of adverse reactions and health complications: Using counterfeit semaglutide can lead to serious adverse reactions, including pancreatitis, gallbladder problems, and kidney issues. These complications can necessitate expensive and extensive medical treatment.
Several news reports detail significant seizures of counterfeit semaglutide products by law enforcement agencies. For example, [insert a link to a relevant news article here, if available]. These seizures highlight the scale of the problem and the need for increased vigilance.
FDA Enforcement Actions Against Off-Brand Ozempic
The FDA employs various strategies to combat the illegal distribution of off-brand semaglutide. These include:
- Issuing warning letters: The FDA sends warning letters to companies and individuals engaged in the illegal manufacturing, distribution, or sale of counterfeit semaglutide products. These letters detail the violations and demand immediate cessation of illegal activities.
- Legal consequences: Manufacturers and distributors of counterfeit medications face severe legal consequences, including hefty fines, product seizures, and even criminal prosecution.
- Significant seizures and enforcement actions: The FDA regularly announces seizures of large quantities of counterfeit semaglutide, demonstrating its commitment to protecting public health. [Insert links to FDA press releases or news articles detailing significant seizures].
- Ensuring safety and efficacy: The FDA rigorously regulates the manufacturing and distribution of legitimate semaglutide products, ensuring they meet stringent quality and safety standards. This contrasts sharply with the unregulated production of counterfeit medications.
Potential Drug Shortages Due to Counterfeit Production
The diversion of resources to counterfeit production significantly impacts the supply of legitimate Ozempic and other semaglutide products. This is a double-edged sword: the increased demand fueled by the popularity of semaglutide is already straining supply chains, and the diversion of ingredients and manufacturing capacity to the counterfeit market exacerbates this issue.
The implications of potential shortages are far-reaching:
- Impact on patients with diabetes: Patients who rely on semaglutide for diabetes management may face difficulty accessing the medication they need, potentially leading to uncontrolled blood sugar levels and associated health complications.
- Increased healthcare costs: Treatment for adverse effects stemming from the use of counterfeit semaglutide places an additional burden on healthcare systems and patients.
- Strain on healthcare systems: Managing the health consequences of counterfeit drug use puts a strain on hospitals, clinics, and healthcare professionals.
The Role of Online Pharmacies and Social Media
Online pharmacies and social media platforms play a significant role in facilitating the sale of off-brand Ozempic and similar products. The anonymity and ease of access offered by these platforms make them attractive to sellers of counterfeit medications. Purchasing medications from unregulated online sources carries significant risks.
- Identifying legitimate online pharmacies: Consumers should only purchase medications from licensed and verified online pharmacies that adhere to FDA regulations. Look for a Verified Internet Pharmacy Practice Sites (VIPPS) accreditation.
Conclusion
The proliferation of off-brand Ozempic presents a serious threat to public health. The FDA's ongoing enforcement efforts are crucial, but more vigilance is needed from healthcare providers and consumers alike. Purchasing semaglutide only from reputable sources and reporting suspicious activity are vital steps in preventing further harm and mitigating potential drug shortages. Remember, prioritizing your health and safety means avoiding the risks associated with off-brand Ozempic and choosing FDA-approved medications. Always consult your doctor before starting any new medication, including semaglutide, and report any suspected counterfeit drugs to the relevant authorities.

Featured Posts
-
 Superalimentos Cual Es El Mejor Para Prevenir Enfermedades Cronicas Este Podria Incluir El Arandano En La Descripcion
May 22, 2025
Superalimentos Cual Es El Mejor Para Prevenir Enfermedades Cronicas Este Podria Incluir El Arandano En La Descripcion
May 22, 2025 -
 Abn Amro De Impact Van Amerikaanse Heffingen Op Nederlandse Voedselexport
May 22, 2025
Abn Amro De Impact Van Amerikaanse Heffingen Op Nederlandse Voedselexport
May 22, 2025 -
 Councillors Wifes Jail Sentence For Threatening Tweet Appeal Awaits
May 22, 2025
Councillors Wifes Jail Sentence For Threatening Tweet Appeal Awaits
May 22, 2025 -
 New Business Hotspots Across The Country An Interactive Map
May 22, 2025
New Business Hotspots Across The Country An Interactive Map
May 22, 2025 -
 Core Weave Inc Crwv A Deep Dive Into Last Weeks Stock Market Performance
May 22, 2025
Core Weave Inc Crwv A Deep Dive Into Last Weeks Stock Market Performance
May 22, 2025
Latest Posts
-
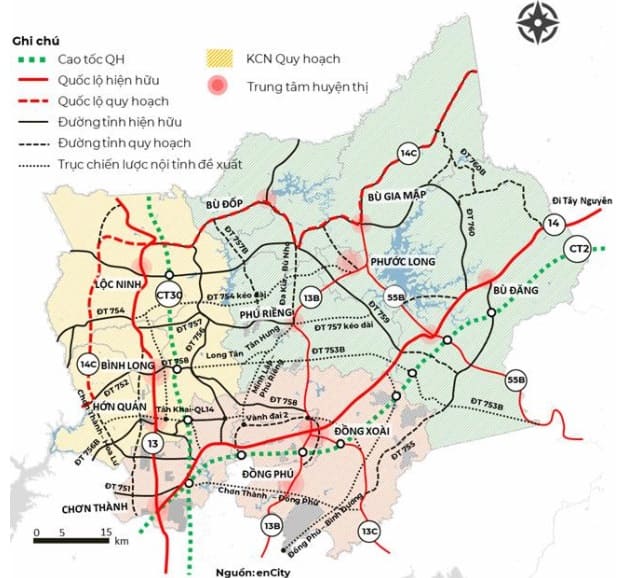 Cau Va Duong Noi Binh Duong Tay Ninh Ten Goi Va Thong Tin Chi Tiet
May 22, 2025
Cau Va Duong Noi Binh Duong Tay Ninh Ten Goi Va Thong Tin Chi Tiet
May 22, 2025 -
 Hai Lo Vuong Tren Dau Noi Usb Chuc Nang Va Giai Dap Thac Mac
May 22, 2025
Hai Lo Vuong Tren Dau Noi Usb Chuc Nang Va Giai Dap Thac Mac
May 22, 2025 -
 The Future Of Core Weave Stock Predictions And Analysis
May 22, 2025
The Future Of Core Weave Stock Predictions And Analysis
May 22, 2025 -
 Nvidias Core Weave Investment Fuels Crwv Stock Price Increase
May 22, 2025
Nvidias Core Weave Investment Fuels Crwv Stock Price Increase
May 22, 2025 -
 Whats Driving Core Weaves Stock Price
May 22, 2025
Whats Driving Core Weaves Stock Price
May 22, 2025
